诺和诺德的Wegovy口服药将测试现金消费者的需求
 AI智能总结
AI智能总结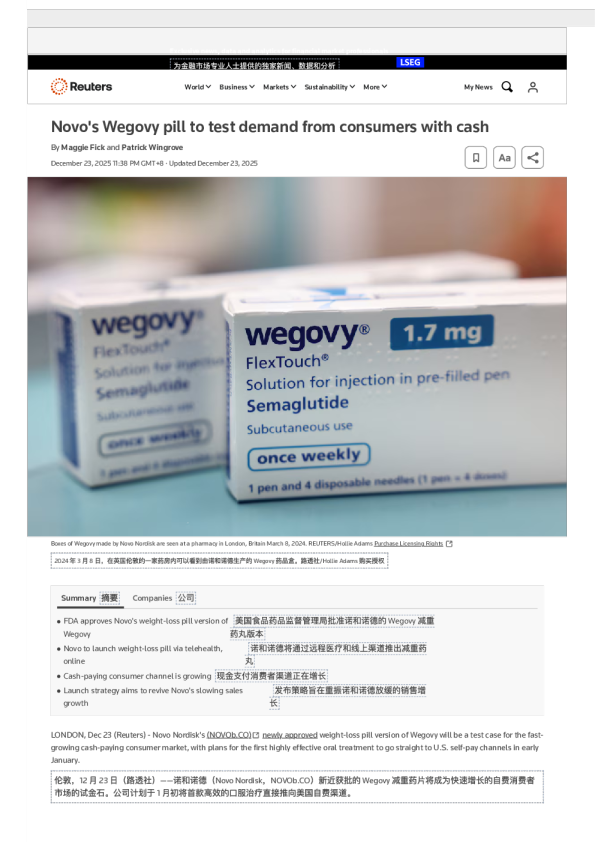
ByMaggie FickandPatrick Wingrove December 23, 202511:38 PM GMT+8·Updated December 23, 2025 2024年3⽉8⽇,在英国伦敦的⼀家药房内可以看到由诺和诺德⽣产的Wegovy药品盒。路透社/Hollie Adams购买授权 Summary摘要Companies公司 FDA approves Novo's weight-loss pill version ofWegovy美国⻝品药品监督管理局批准诺和诺德的Wegovy减重药丸版本Novo to launch weight-loss pill via telehealth,online诺和诺德将通过远程医疗和线上渠道推出减重药丸Cash-paying consumer channel is growing现⾦⽀付消费者渠道正在增⻓Launch strategy aims to revive Novo's slowing salesgrowth发布策略旨在重振诺和诺德放缓的销售增⻓ LONDON, Dec 23 (Reuters) - Novo Nordisk's(NOVOb.CO)newly approvedweight-loss pill version of Wegovy will be a test case for the fast-growing cash-paying consumer market, with plans for thefirst highly effective oral treatment to go straight to U.S. self-pay channels in earlyJanuary. 伦敦,12⽉23⽇(路透社)——诺和诺德(Novo Nordisk,NOVOb.CO)新近获批的Wegovy减重药⽚将成为快速增⻓的⾃费消费者市场的试⾦⽯。公司计划于1⽉初将⾸款⾼效的⼝服治疗直接推向美国⾃费渠道。 The pill was granted U.S. Food and Drug Administration approval on Monday, a boost for Danish drugmaker Novo as it looks to claw background lost to U.S. rival Eli Lilly(LLY.N). 该药⽚周⼀获得美国⻝品药品监督管理局批准,这对丹⻨制药商诺和诺德⽽⾔是⼀⼤利好,因为它正试图夺回被美国竞争对⼿礼来(Eli Lilly,LLY.N)抢⾛的市场份额。 Keep up with the latest medical breakthroughs and healthcare trends with the Reuters Health Roundsnewsletter.Sign uphere. 通过《路透社健康纵览》新闻通讯,及时了解最新的医学突破和医疗健康趋势。点击此处订阅。 Novo Nordisk shares jumped nearly 10% on Tuesday. Lilly's shares wereflat. 诺和诺德股价周⼆⼤涨近10%。礼来股价持平。 A key part of making it a success will be attracting cash-paying consumers, a stark shift from a business model where drug pricing ismanaged through health insurance plans, which has dominated for decades. Under a deal with the Trump administration in November, Novo and Lillyagreed to sellstarter doses of their weight‑loss pills, if approved, for$149 a month to U.S. Medicare and Medicaid patients and cash-paying customers who cannot get insurance coverage for the medications. 根据11⽉与特朗普政府达成的⼀项协议,诺和诺德和礼来同意,如果其减肥药获得批准,将以每⽉149美元的价格向美国医保(Medicare)和医疗补助(Medicaid)患者,以及⽆法获得相关药物保险覆盖的⾃费消费者出售起始剂量。 The Trump administration said its TrumpRx site, launching in January, will list oral and injectable weight-loss drugs at an average startingprice of $350 a month. 特朗普政府表示,其将于1⽉上线的TrumpRx⽹站将以平均每⽉350美元的起始价格列出⼝服和注射型减肥药。 "We have a self-pay offer from day one for U.S. patients," David Moore, Novo's executive vice president for U.S. operations, told Reutersahead of the pill's approval. “从第⼀天起,我们就为美国患者提供⾃费⽅案,”诺和诺德美国业务执⾏副总裁⼤卫·摩尔在该药⽚获批前对路透表示。 Novo declined to reveal the expected cash prices for higher doses of its Wegovy pill, saying they will be made available at launch. Lilly hassaid higher doses of its obesity pill, if approved, would be capped at $399 a month for repeat cash purchasers. 诺和诺德拒绝透露其Wegovy⼝服药更⾼剂量的预期⾃费价格,称将在上市时公布。礼来表示,如果其肥胖症⼝服药获批,更⾼剂量对重复⾃费购买者的⽉度价格将上限设为399美元。 Novo plans to launch the pill on multiple channels - including retail pharmacies such as CVS(CVS.N)and Walmart(WMT.O), onlineplatforms like GoodRx(GDRX.O)and telehealth partners including Ro and WeightWatchers - so people can start treatment withoutwaiting for insurance coverage, Moore said. 摩尔称,诺和诺德计划通过多种渠道推出该药⽚——包括CVS(CVS.N)和沃尔玛(WMT.O)等零售药房、GoodRx(GDRX.O)等在线平台,以及Ro和WeightWatchers等远程医疗合作伙伴——以便⼈们⽆需等待保险覆盖即可开始治疗。 The share of U.S. Wegovy prescriptions - until now injectable versions - via self-pay channels has jumped from about 5% to double digits thisyear, Moore said. 摩尔表示,今年通过⾃费渠道获得的美国Wegovy处⽅(迄今为⽌均为注射剂)的占⽐已从约5%跃升⾄两位数。 诺和诺德的这款药⽚将以四种剂量出售,分别为1.5毫克、4毫克、9毫克以及⻓期使⽤的更⾼剂量25毫克。 NEW STRATEGY FOR A DRUG LAUNCH ⼀种全新的药物上市策略 The focus on cash-paying consumers aims to revive Novo's slowing sales growth and turbocharge the next stage of expansion for the widermarket. Novo has lost hundreds of billions of dollars in market capitalisation since mid-2024 amid rising competition. 将重点放在⾃掏腰包的消费者身上,旨在重振诺和诺德放缓的销售增⻓,并为更⼴泛市场的下⼀阶段扩张注⼊强劲动⼒。⾃2024年年中以来,在竞争加剧的背景下,诺和诺德的市值已蒸发数千亿美元。 "We've never launched this way before," Moore said. “我们以前从未以这种⽅式推出过产品,”穆尔说。 In the past, "the mindset was more traditional – the product is available, you wait for insurers to cover it, and it's at the retail pharmacy," hesaid. 他表示,过去“思维⽅式更为传统——产品上市后,等待保险公司将其纳⼊报销范围,然后在零售药房销售”。 Novo is facing intensifying competition from Lilly's rival obesity drug Zepbound, known outside the U.S. as Mounjaro, and pressure fromcheaper, unapproved compounded versions of semaglutide, the active ingredient in the Wegovy injection and pill. 诺和诺德正⾯临来⾃礼来公司竞争性肥胖症药物Zepbound的激烈竞争,该药在美国以外被称为Mounjaro,同时也承受着来⾃更便宜、未经批准的复⽅司美格鲁肽版本的压⼒。司美格鲁肽是Wegovy注射剂和药⽚中的活性成分。 Lilly is awaiting U.S. approval for itsweight-loss pill, and has said a decision could come as early as March. 礼来正在等待其减重药⽚在美国获批,并表示相关决定最早可能于3⽉作出。 Novo hopes the once-daily oral dose of Wegovy could be a turning point in attracting people who were not motivated to start treatment withGLP‑1 injections. 诺和诺德希望每⽇⼀次⼝服的Wegovy能够成为⼀个转折点,吸引那些此前缺乏动⼒开始接受GLP‑1注射治疗的⼈。 WEIGHT-LOSS PILLS AN 'EASIER ON-RAMP' 减肥药被称为“更容易的⼊⻔⽅式” Novo's U.S. medical head, Dr. Jason Brett, told Reuters the pill could broaden access by givin




