世界卫生组织所列机构WLA名单
 AI智能总结
AI智能总结
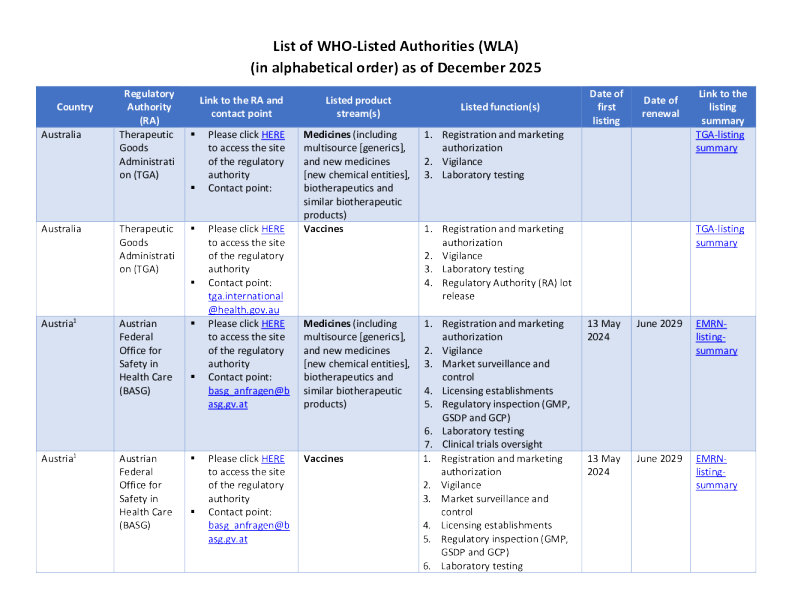
1Evaluated as part of the European Medicines Regulatory Network 2The European Medicines Regulatory Network is composed of the European Commission, the European Medicines Agency and the regulatory authorities of thefollowing EU/EEA-EFTA countries: Austria, Belgium, Bulgaria, Croatia, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland,Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden. Inaddition, the European Directorate for the Quality of Medicines & HealthCare (EDQM) coordinates laboratory testing and lot release related activities for theEMRN.